ORAL Strategy is the First Trial to Compare a JAK Inhibitor,
XELJANZ, as Monotherapy or in Combination with Methotrexate (MTX) versus
Humira® (adalimumab) plus MTX
NEW YORK–(BUSINESS WIRE)– Pfizer Inc. (NYSE:PFE) announced today detailed results from ORAL
Strategy, a head-to-head, noninferiority Phase 3b/4 study of XELJANZ®
(tofacitinib citrate) 5 mg twice daily (BID) as monotherapy or in
combination with methotrexate (MTX) compared to Humira® plus
MTX in the treatment of moderate to severe rheumatoid arthritis (RA).
ORAL Strategy also compared XELJANZ monotherapy to XELJANZ in
combination with MTX. The study results were published online
in The Lancet and will be presented during an oral session at the
EULAR Annual European Congress of Rheumatology in Madrid, Spain (16
June).
This Smart News Release features multimedia. View the full release here:
http://www.businesswire.com/news/home/20170616005164/en/
Clik here to view.
“Our extensive RA clinical development program has demonstrated the
overall efficacy and safety of XELJANZ with or without methotrexate in
patients living with moderate to severe RA. ORAL Strategy is a bold
study that directly compared XELJANZ as a monotherapy or in combination
with methotrexate to Humira in combination with methotrexate,” said
Michael Corbo, Chief Development Officer, Inflammation & Immunology,
Pfizer Global Product Development. “The totality of the ORAL Strategy
results add to body of evidence for XELJANZ and further demonstrates
Pfizer’s commitment to putting patients first by helping physicians make
informed treatment decisions.”
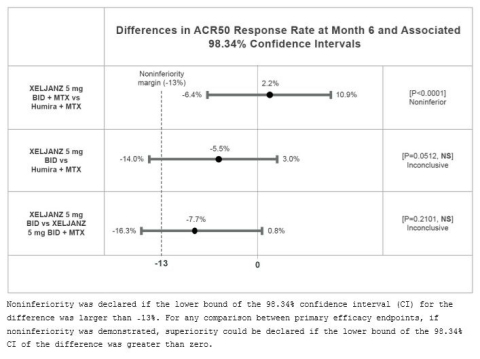
Efficacy Results
The percentage of patients achieving an ACR50 response at Month 6, the
primary efficacy endpoint, for each arm include:
- XELJANZ 5 mg BID plus MTX: 46.0% (n=173)
- XELJANZ 5 mg BID monotherapy: 38.3% (n=147)
- Humira 40 mg every other week (EOW) plus MTX: 43.8% (n=169)
Refer to data plot showing “Differences in ACR50 Response Rate at Month
6.”
“As expected, XELJANZ in combination with methotrexate provided similar
ACR50 response rates to Humira plus methotrexate,” said Dr. Roy
Fleischmann, study author and clinical professor in the Department of
Internal Medicine at the University of Texas Southwestern Medical Center
and Co-Medical Director, Metroplex Clinical Research Center. “Although
XELJANZ monotherapy did not demonstrate noninferiority to either
combination arm, the clinical responses observed are reflective of those
in the Phase 3 clinical program and affirm our understanding that
XELJANZ is an important option both in combination with MTX and as
monotherapy for patients who do not respond to or are intolerant to
methotrexate.”
Safety Results
The safety findings in ORAL Strategy were consistent with the known
adverse events (AEs) profile for XELJANZ. The most frequently reported
AEs for each study group were upper respiratory tract infections,
alanine aminotransferase elevation, nasopharyngitis, urinary tract
infections and nausea. Overall AEs rates were comparable between
treatment arms; the majority of AEs were mild to moderate in severity.
Rates of serious AEs (SAEs) and discontinuations due to AEs were
generally similar between treatment arms. Over the course of the study,
the following percentages of patients experienced AEs and serious AEs
across treatment groups:
AEs
- XELJANZ 5 mg BID plus MTX: 61.4% (n= 231)
- XELJANZ 5 mg BID monotherapy: 58.9% (n=226)
- Humira 40 mg EOW plus MTX: 65.5% (n= 253)
SAEs
- XELJANZ 5 mg BID plus MTX: 7.2% (n=27)
- XELJANZ 5 mg BID monotherapy: 9.1% (n=35)
- Humira 40 mg EOW plus MTX: 6.2% (n=24)
Top-line results for ORAL Strategy were announced
in February 2017.
About Rheumatoid Arthritis (RA)
RA is a chronic, inflammatory autoimmune disease that affects
approximately 17.6 million people worldwide and 1.6 million people in
the U.S. It causes a range of symptoms, including pain and swelling in
the joints, particularly those in the hands, feet and knees, which may
lead to joint damage and eventual disability. RA can be treated with
various types of medications, including steroids, conventional synthetic
disease-modifying antirheumatic drugs (csDMARDs) and biologic
disease-modifying antirheumatic drugs (bDMARDs). Many physicians use
combination therapy with MTX when treating patients with moderate to
severe RA. However, some patients discontinue their MTX, which may
result in reduced efficacy of these treatments regimens.
About XELJANZ (tofacitinib citrate) and XELJANZ XR (tofacitinib
citrate) extended-release
XELJANZ®/XELJANZ XR® (tofacitinib citrate) is a
prescription medicine called a Janus kinase (JAK) inhibitor. XELJANZ is
approved in more than 80 countries around the world for the treatment of
moderately to severely active rheumatoid arthritis (RA). Since it was
first approved in the United States in 2012, XELJANZ has been prescribed
to more than 90,000 patients worldwide. XELJANZ XR is the first
once-daily oral JAK inhibitor approved for the treatment of moderately
to severely active RA in eight countries around the world.
XELJANZ/XELJANZ XR U.S. Label Information
XELJANZ (tofacitinib citrate)/XELJANZ XR (tofacitinib citrate) is a
prescription medicine called a Janus kinase (JAK) inhibitor.
XELJANZ/XELJANZ XR is used to treat adults with moderately to severely
active rheumatoid arthritis in which methotrexate did not work well.
XELJANZ/XELJANZ XR may be used as a single agent or in combination with
methotrexate (MTX) or other non-biologic disease-modifying antirheumatic
drugs (DMARDs). Use of XELJANZ/XELJANZ XR in combination with biologic
DMARDs or potent immunosuppressants, such as azathioprine and
cyclosporine, is not recommended.
-
It is not known if XELJANZ/XELJANZ XR is safe and effective in people
with hepatitis B or C. - XELJANZ/XELJANZ XR is not for people with severe liver problems.
-
It is not known if XELJANZ/XELJANZ XR is safe and effective in
children.
Important Safety Information
-
XELJANZ/XELJANZ XR can lower the ability of the immune system to
fight infections. Some people can have serious infections while taking
XELJANZ/XELJANZ XR, including tuberculosis (TB), and infections caused
by bacteria, fungi, or viruses that can spread throughout the body.
Some people have died from these infections. Healthcare providers
should test patients for TB before starting XELJANZ/XELJANZ XR, and
monitor them closely for signs and symptoms of TB and other infections
during treatment. People should not start taking XELJANZ/XELJANZ XR if
they have any kind of infection unless their healthcare provider tells
them it is okay. - People may be at a higher risk of developing shingles.
-
XELJANZ/XELJANZ XR may increase the risk of certain cancers by
changing the way the immune system works. Lymphoma and other cancers,
including skin cancers, can happen in patients taking XELJANZ/XELJANZ
XR. -
The risks and benefits of treatment should be considered prior to
initiating XELJANZ/XELJANZ XR in patients with chronic or recurrent
infection; who have been exposed to tuberculosis; with a history of a
serious or an opportunistic infection; who have resided or traveled in
areas of endemic tuberculosis or endemic mycoses; or with underlying
conditions that may predispose them to infection. -
Viral reactivation, including cases of herpes virus reactivation
(e.g., herpes zoster), was observed in clinical studies with XELJANZ. -
Use of live vaccines should be avoided concurrently with
XELJANZ/XELJANZ XR. Update immunizations in agreement with current
immunization guidelines prior to initiating XELJANZ/XELJANZ XR therapy. -
Some people who have taken XELJANZ with certain other medicines to
prevent kidney transplant rejection have had a problem with certain
white blood cells growing out of control (Epstein Barr
virus-associated post-transplant lymphoproliferative disorder). -
Some people taking XELJANZ/XELJANZ XR can get tears in their stomach
or intestines. This happens most often in people who also take
nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or
methotrexate. -
XELJANZ/XELJANZ XR should be used with caution in patients who may be
at increased risk for gastrointestinal perforation (e.g., patients
with a history of diverticulitis), or who have a narrowing within
their digestive tract. Patients should tell their healthcare provider
right away if they have fever and stomach-area pain that does not go
away or a change in bowel habits. -
XELJANZ/XELJANZ XR can cause changes in certain lab test results
including low blood cell counts, increases in certain liver tests, and
increases in cholesterol levels. Healthcare providers should do blood
tests before starting patients on XELJANZ/XELJANZ XR and while they
are taking XELJANZ/XELJANZ XR, to check for these side effects. Normal
cholesterol levels are important to good heart health. Healthcare
providers may stop XELJANZ/XELJANZ XR treatment because of changes in
blood cell counts or liver test results. -
Use of XELJANZ/XELJANZ XR in patients with severe hepatic impairment
is not recommended. -
Patients should tell their healthcare providers if they plan to become
pregnant or are pregnant.
It is not known if XELJANZ/XELJANZ XR will harm an unborn baby. To
monitor the outcomes of pregnant women exposed to XELJANZ/XELJANZ XR, a
registry has been established. Physicians are encouraged to register
patients and pregnant women are encouraged to register themselves by
calling 1-877-311-8972.
-
Patients should tell their healthcare providers if they plan to
breastfeed or are breastfeeding. Patients and their healthcare
provider should decide if they will take XELJANZ/XELJANZ XR or
breastfeed. They should not do both. -
In carriers of the hepatitis B or C virus (viruses that affect the
liver), the virus may become active while using XELJANZ/XELJANZ XR.
Healthcare providers may do blood tests before and during treatment
with XELJANZ/XELJANZ XR. -
Common side effects include upper respiratory tract infections (common
cold, sinus infections), headache, diarrhea, and nasal congestion,
sore throat, and runny nose (nasopharyngitis).
Please click the direct link to the full US Prescribing Information for
XELJANZ/XELJANZ XR, including Boxed Warning and Medication Guide: http://labeling.pfizer.com/ShowLabeling.aspx?id=959.
Pfizer Inc.: Working together for a healthier world®
At Pfizer, we apply science and our global resources to bring therapies
to people that extend and significantly improve their lives. We strive
to set the standard for quality, safety and value in the discovery,
development and manufacture of health care products. Our global
portfolio includes medicines and vaccines as well as many of the world’s
best-known consumer health care products. Every day, Pfizer colleagues
work across developed and emerging markets to advance wellness,
prevention, treatments and cures that challenge the most feared diseases
of our time. Consistent with our responsibility as one of the world’s
premier innovative biopharmaceutical companies, we collaborate with
health care providers, governments and local communities to support and
expand access to reliable, affordable health care around the world. For
more than 150 years, we have worked to make a difference for all who
rely on us. We routinely post information that may be important to
investors on our website at www.pfizer.com.
In addition, to learn more, please visit us on www.pfizer.com
and follow us on Twitter at @Pfizer
and @PfizerNews,
LinkedIn,
YouTube
and like us on Facebook at Facebook.com/Pfizer.
DISCLOSURE NOTICE: The information contained in this release is as of
June 16, 2017. Pfizer assumes no obligation to update forward-looking
statements contained in this release as the result of new information or
future events or developments.
This release contains forward-looking information about XELJANZ
(tofacitinib citrate) that involves substantial risks and uncertainties
that could cause actual results to differ materially from those
expressed or implied by such statements. Risks and uncertainties
include, among other things, the uncertainties inherent in research and
development, including, without limitation, the ability to meet
anticipated trial commencement and completion dates and regulatory
submission dates, as well as the possibility of unfavorable clinical
trial results, including unfavorable new clinical data and additional
analyses of existing clinical data; uncertainties regarding the
commercial success of XELJANZ and XELJANZ XR; uncertainties regarding
the commercial impact of the results of the ORAL Strategy trial; whether
and when any other applications for XELJANZ or XELJANZ XR may be filed
with regulatory authorities in any jurisdictions; whether and when
regulatory authorities in any jurisdictions may approve any such
applications and/or any other applications that are pending or may be
filed for XELJANZ or XELJANZ XR, which will depend on the assessment by
such regulatory authorities of the benefit-risk profile suggested by the
totality of the efficacy and safety information submitted; decisions by
regulatory authorities regarding labeling and other matters that could
affect the availability or commercial potential of XELJANZ and XELJANZ
XR; and competitive developments.
A further description of risks and uncertainties can be found in
Pfizer’s Annual Report on Form 10-K for the fiscal year ended December
31, 2016 and in its subsequent reports on Form 10-Q, including in the
sections thereof captioned “Risk Factors” and “Forward-Looking
Information and Factors That May Affect Future Results”, as well as in
its subsequent reports on Form 8-K, all of which are filed with the U.S.
Securities and Exchange Commission and available at www.sec.gov and www.pfizer.com.
Image may be NSFW.
Clik here to view.
View source version on businesswire.com: http://www.businesswire.com/news/home/20170616005164/en/
Contacts
Pfizer
Media:
Steven Danehy, +1 978-273-3946
Steven.Danehy@pfizer.com
or
Investors:
Chuck
Triano, +1 212-733-3901
Charles.E.Triano@pfizer.com
Source: Pfizer Inc.
Cet article Pfizer Announces Results from XELJANZ®
(tofacitinib citrate) ORAL Strategy Study Published in The Lancet
and Presented at the EULAR Annual Congress est apparu en premier sur EEI-BIOTECHFINANCES.

